Compliance Guide for Beauty Salons and Aesthetic Clinics in the United Kingdom (2026 Edition)
1. Executive Summary
The UK beauty and aesthetics industry is currently undergoing its most significant regulatory transformation in decades. As of 2026, the “Wild West” era of unregulated cosmetic procedures has largely come to an end. This guide outlines the mandatory compliance requirements for business owners and practitioners, incorporating the latest licensing schemes, consumer protection laws, and health and safety standards.
2. The National Licensing Scheme (England)
Under the Health and Care Act 2022, the UK government has implemented a national licensing scheme for non-surgical cosmetic procedures in England.
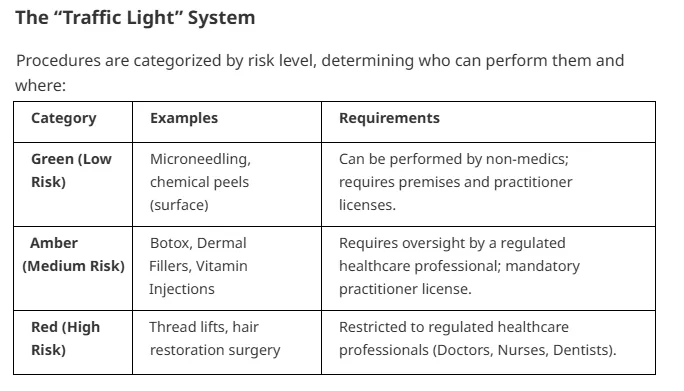
Dual Licensing Requirement
Most practitioners now require two distinct licenses:
1. Practitioner License: Demonstrating individual competence and qualification (typically Level 7 for injectables).
2. Premises License: Ensuring the clinic environment meets clinical hygiene and safety standards.
3. Consumer Protection: DMCCA 2024
The Digital Markets, Competition and Consumers Act (DMCCA) 2024, which came into full effect in April 2025, has introduced strict rules for beauty businesses.
Fake Reviews: It is now a criminal offense to solicit, post, or fail to take reasonable steps to prevent fake reviews.
Subscription Contracts: If your salon offers “membership” or “subscription” packages, you must provide clear pre-contract information, send renewal reminders, and offer a simple “one-click” cancellation process.
Price Transparency: All prices must be inclusive of VAT and clearly displayed. “Drip pricing” (adding mandatory fees at the end of a booking) is prohibited.
4. Core Health & Safety Compliance
Standard health and safety laws remain the foundation of salon compliance.
Health and Safety at Work Act 1974
You have a legal “duty of care” to ensure the safety of staff, clients, and visitors. This includes conducting regular Risk Assessments.
COSHH (Control of Substances Hazardous to Health)
Salons use many hazardous substances (hair dyes, nail monomers, cleaning chemicals). You must:
- Maintain a COSHH Register.
- Keep Safety Data Sheets (SDS) for all products.
- Provide appropriate Personal Protective Equipment (PPE).
RIDDOR
The Reporting of Injuries, Diseases and Dangerous Occurrences Regulations require you to report serious workplace accidents, occupational diseases, and specified “dangerous occurrences” to the HSE.
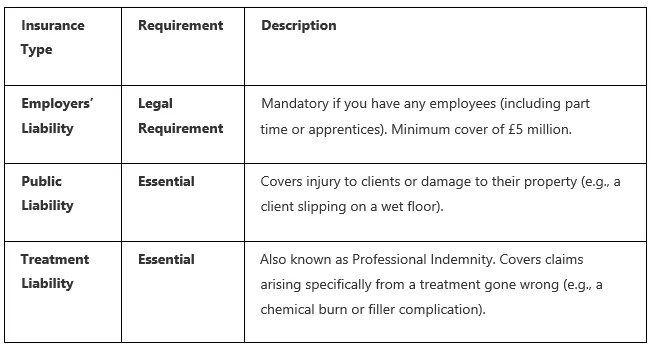
6. Mandatory Insurance
Operating without the correct insurance is a major compliance failure and a significant financial risk.
7. Data Protection & Privacy (GDPR)
Salons handle sensitive personal data, including medical histories and contact details.
ICO Registration: Most salons must register with the Information Commissioner’s Office (ICO) and pay a data protection fee.
Privacy Policy: You must have a clear policy explaining how you collect, store, and use client data.
Consent: Explicit consent is required for marketing and for processing “special category” data (health information).
8. Energy-Based Devices (IPL, Laser, Plasma)
The use of energy-based devices is strictly regulated under the UK Medical Devices Regulations (UK MDR) 2002 and the new 2025 licensing framework.
IPL and Laser Treatments
Device Compliance: All lasers and IPL machines must be UKCA marked (or CE marked if placed on the market before July 2023). Using uncertified “grey market” devices is a major compliance breach.
Local Authority Licensing: In many jurisdictions (e.g., London), Laser and IPL treatments require a specific Special Treatment License.
Core Standards: Practitioners must hold a minimum of Level 4 qualification in Laser and Light treatments.
Safety Officers: Clinics should appoint a Laser Protection Adviser (LPA) and a Laser Protection Supervisor (LPS) to oversee safety protocols and “Local Rules.”
Plasma Skin Tightening (Fibroblast)
Classification: Plasma devices (which create an electrical arc to sublimate skin) are high-risk. Under the 2025 scheme, they are categorized as Amber or Red depending on the depth of treatment.
Training: Requires specialized training and often a Level 4 or higher qualification in skin rejuvenation.
Insurance: Many standard beauty policies exclude plasma fibroblast; you must ensure your Treatment Liability specifically covers “thermal skin sublimation” or “plasma fibroblast.”
PEMF (Pulsed Electromagnetic Field) Therapy
Regulation: PEMF devices used for wellness and recovery are regulated as medical devices if they make medical claims.
Safety: While non-invasive, PEMF has strict contraindications (e.g., pacemakers, pregnancy, epilepsy). Compliance requires a robust client screening process.
Insurance: Requires specific “Electromagnetic Therapy” or “Pulsing Insurance” cover.
9. Specific Treatment Regulations
Botox and Prescription-Only Medicines (POMs)
Prescription: Botox is a POM and must be prescribed by a qualified prescriber (Doctor, Dentist, or Nurse Prescriber) after a face-to-face consultation.
Advertising: It is illegal to advertise Botox to the public (e.g., on Instagram or price lists). You can advertise “consultations for fine lines and wrinkles” instead.
Dermal Fillers
Age Restriction: It is illegal to administer fillers to anyone under 18 for cosmetic purposes.
Licensing: Fillers are now strictly regulated under the new 2025 licensing scheme.
Clinical Waste
You must have a contract with a licensed waste carrier for the disposal of:
Sharps (needles, blades).
Infectious Waste (blood-contaminated items).
Chemical Waste (unused dyes or solvents)
9. 2026 Compliance Checklist
Use this checklist to ensure your business is fully compliant:
Business Structure: Registered with Companies House or HMRC as a sole trader.
Licensing: Applied for/Renewed Practitioner and Premises licenses (England).
Local Council: Checked if an MST license is required in your borough. Insurance: Valid Employers’, Public, and Treatment Liability policies in place.
Risk Assessment: Completed and documented for all treatments and the premises.
COSHH: Register updated with latest Safety Data Sheets.
GDPR: Registered with the ICO and privacy policy displayed.
Contracts: Employment contracts and client consent forms reviewed for DMCCA compliance.
Training: All staff certificates are up to date and displayed.
Waste: Valid clinical waste disposal contract in place.
10. Useful Resources
NHBF (National Hair & Beauty Federation): www.nhbf.co.uk
JCCP (Joint Council for Cosmetic Practitioners): www.jccp.org.uk
HSE (Health and Safety Executive): www.hse.gov.uk
ICO (Information Commissioner’s Office): www.ico.org.uk
Disclaimer: This guide is for informational purposes only and does not constitute legal advice. Regulations can vary by region (Scotland, Wales, Northern Ireland) and local authority.
